ToxEraser FCM
May 2, 2022SILIFOOD was developed to support a fast risk assessment of non-evaluated Food Contact Material (FCM) substances.
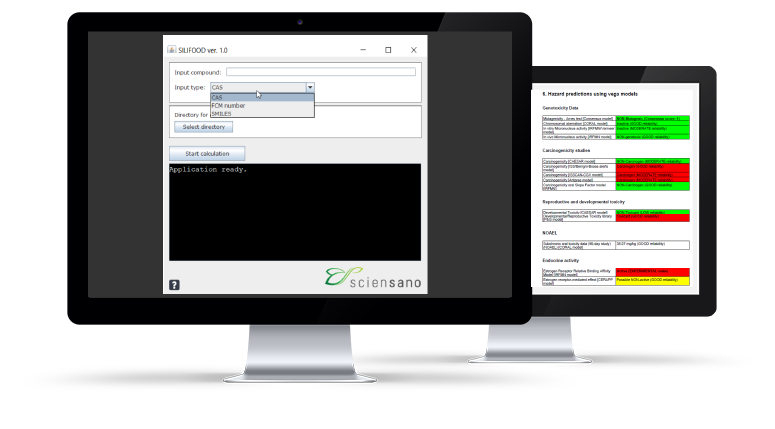
Within the SILIFOOD project (RF21/6349), funded by the Belgian government, the SILIFOOD application was developed to support a fast risk assessment of non-evaluated Food Contact Material (FCM) substances.
The application combines existing toxicological data with (quantitative) structure-activity relationship ((Q)SAR) predictions and has been developed specifically for non-evaluated single organic FCM substances.
SILIFOOD tool was developed by Sciensano in collaboration with Kode Chemoinformatics and Istituto Di richerche Farmacologiche Mario Negri
SILIFOOD tool is freely available for download
The software is a stand-alone, publicly accessible software.
To install the application, the user needs to download the file.zip on its computer. Then, the zipped file has to be unpacked by selecting the file and clicking on ‘Extract all’. The application is started after a double click on the file ‘starter’ in the unpacked application folder.
Zip file contains the tool and the manual (user guide) explaining how to use it.
SILIFOOD Screenshots
Use of the application
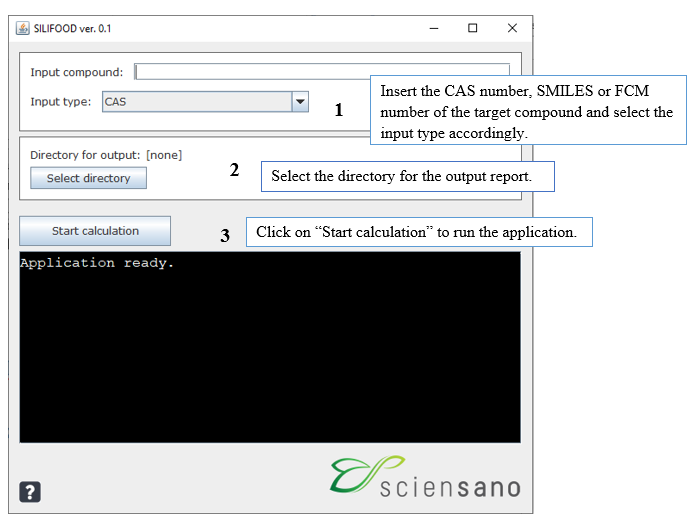
The application can be run in just three steps:
- Insert the ‘Input compound’ (chemical identifier) and select the ‘Input type’: The input type can be the CAS number, the SMILES or the FCM number of the target compound.
- Select the directory for the output on your computer by clicking on ‘Select directory’: The pdf report generated by the tool will appear in the selected folder.
- Click on ‘Start calculation’ to run the workflow, and the report will be generated.
The application will collect information from existing databases and lists and provide VEGA predictions for toxicological endpoints relevant for FCM substances.
Information sources and type of data
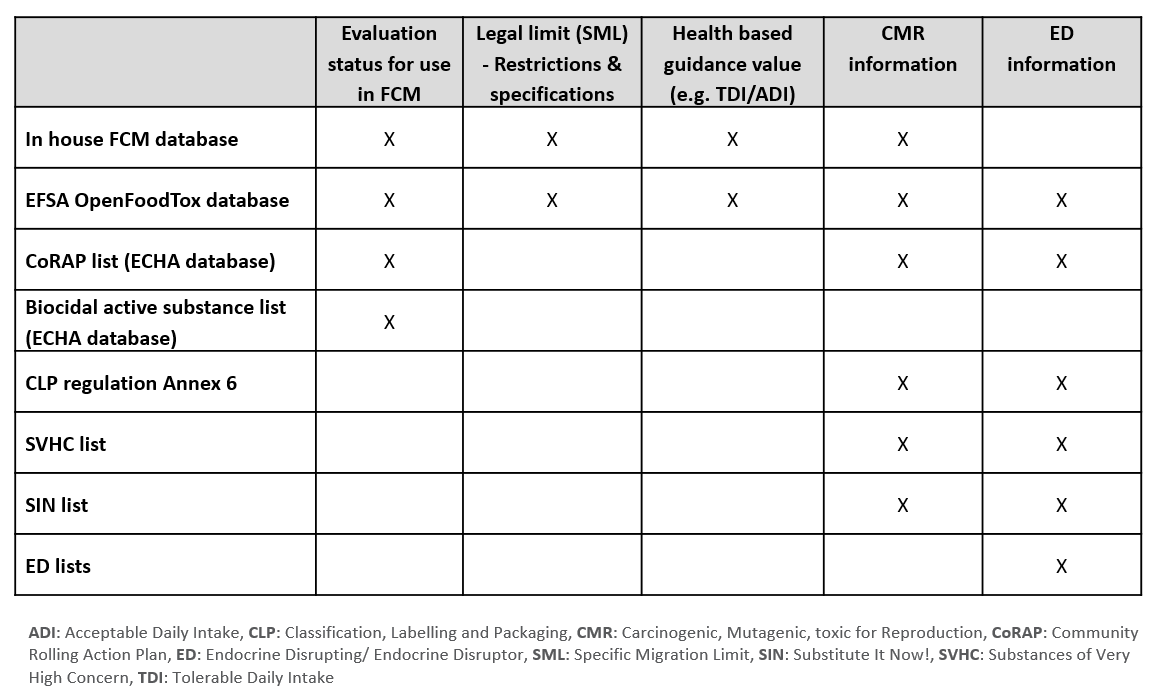
The lists and databases that are searched by the SILIFOOD tool comprise the Sciensano in-house FCM database, the Open Food Tox database (EFSA), the Community Rolling Action Plan (CoRAP) list and the biocidal active substance list (ECHA), Annex 6 of the CLP regulation, the Substances of Very High Concern (SVHC) list, Substitute It Now! (SIN) list and the Endocrine Disruptor (ED) lists.
The type of information retrieved varies by source and comprises regulatory information (Specific Migration Limit (SML), restrictions of use), health based guidance values (Tolerable Daily Intake (TDI)/ Acceptable Daily Intake (ADI)) as well as Carcinogenic, Mutagenic or toxic for Reproduction (CMR) properties and Endocrine Disrupting (ED) activity for the substance.
VEGA models included in the SILIFOOD application
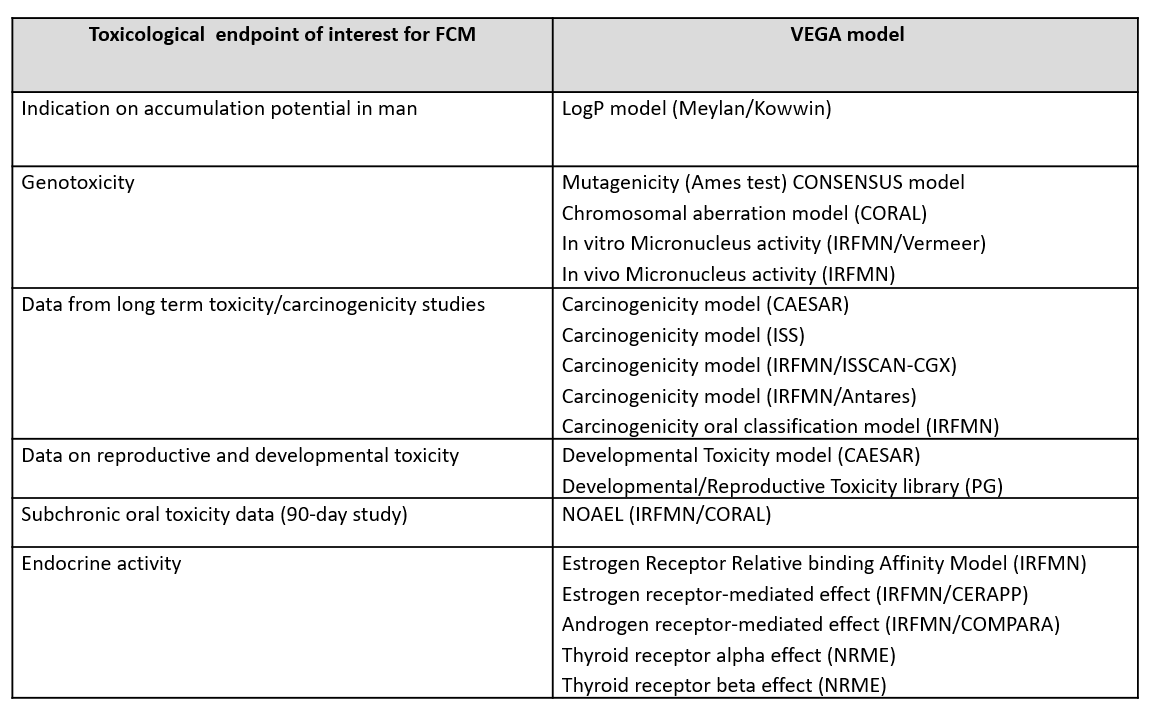
The VEGA models were selected in line with the information requirements for plastic FCM substances and include models for genotoxicity, carcinogenicity, reproductive and developmental toxicity, subchronic toxicity and endocrine activity. A model for the logP values was also integrated to provide an indication for the accumulation potential of the substance.
Report
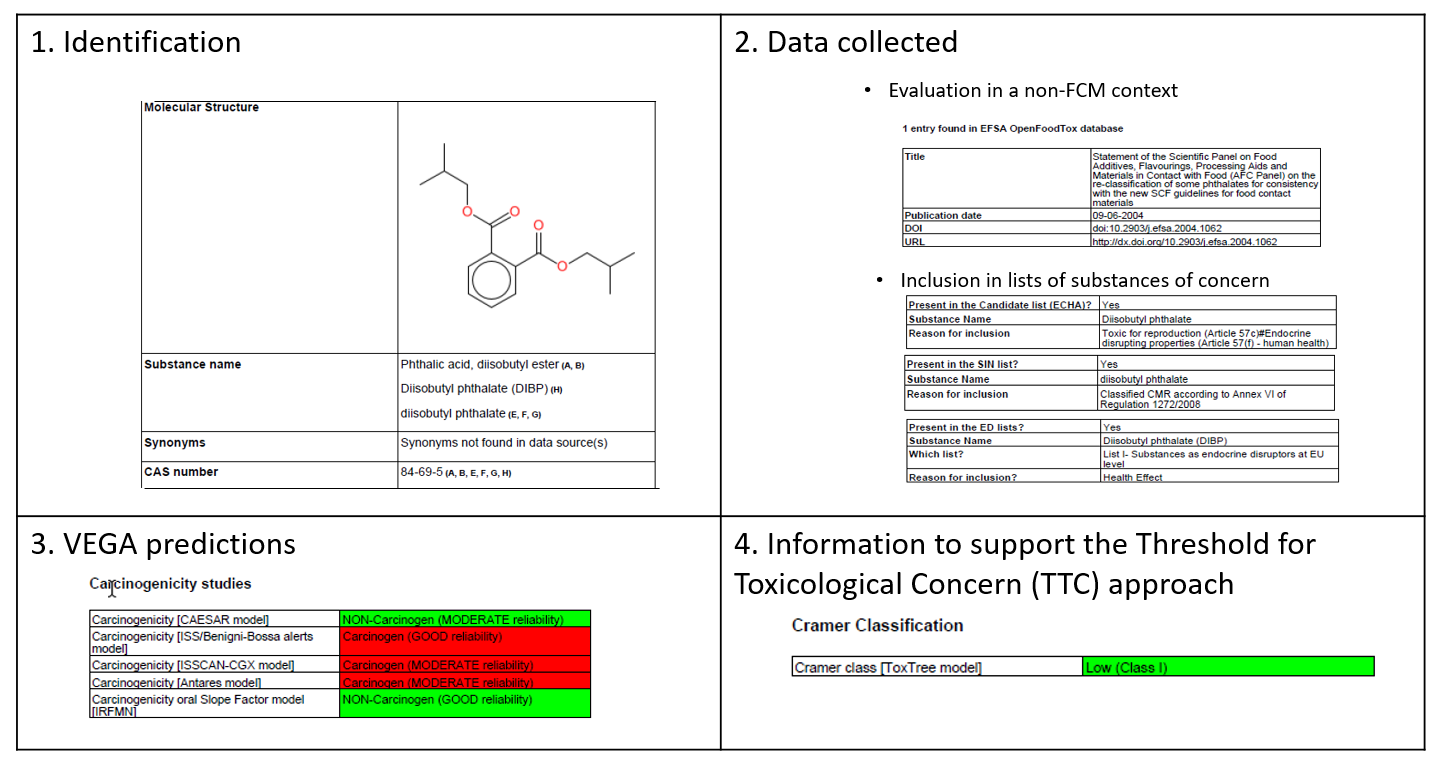
The pdf report consists of 4 parts. The first part relates to the identification of the substance. In Part 2, an overview of the data collected per information source is provided. Part 3 summarizes the VEGA predictions. A color code is used to reflect the level of concern for the hazard. The last part of the report provides the predicted Cramer Class as information to support the application of the Threshold for Toxicological Concern (TTC) approach.
By applying the SILIFOOD tool, the user can collect existing and predicted information for the substance of interest in a single report. The user can decide how to use the data to support a fast evaluation of the risk associated with the presence of the substance in food after migration from FCM.