ToxEraser FCM
May 2, 2022
aiQSAR
October 20, 2020Within the LIFE VERMEER project , a new software system for cosmetics has been designed allowing an overall evaluation of cosmetic ingredients and providing detailed investigation of cosmetics risk scenarios.
The software, freely available, is intended to provide a one-shop system for the risk assessment of cosmetic products. It covers both exposure and hazard.
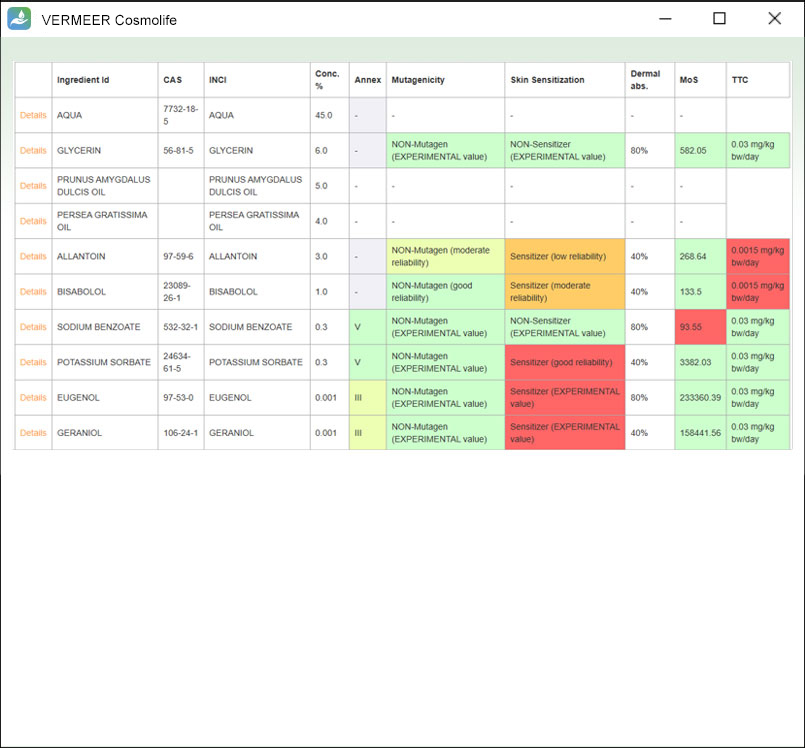
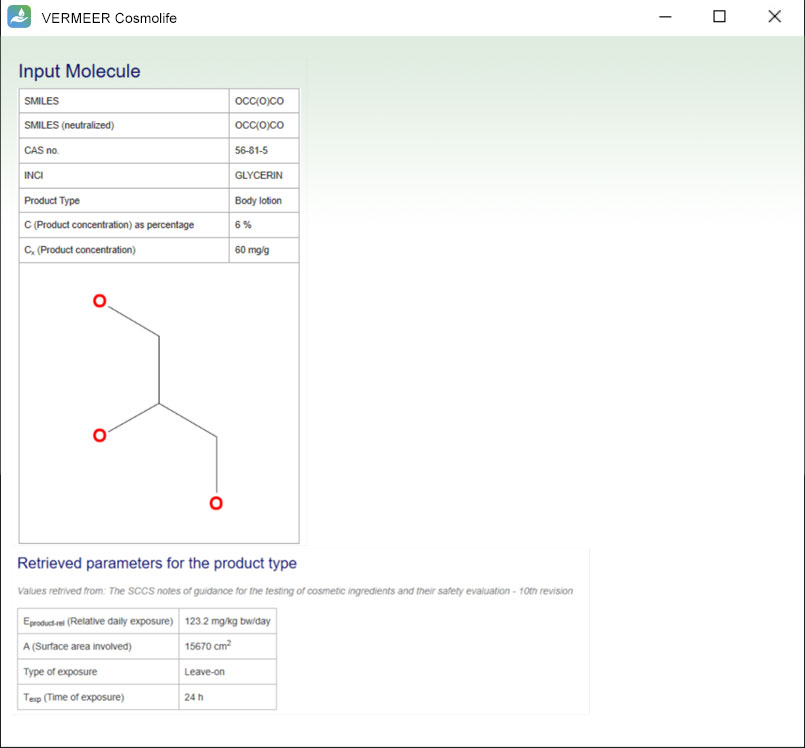
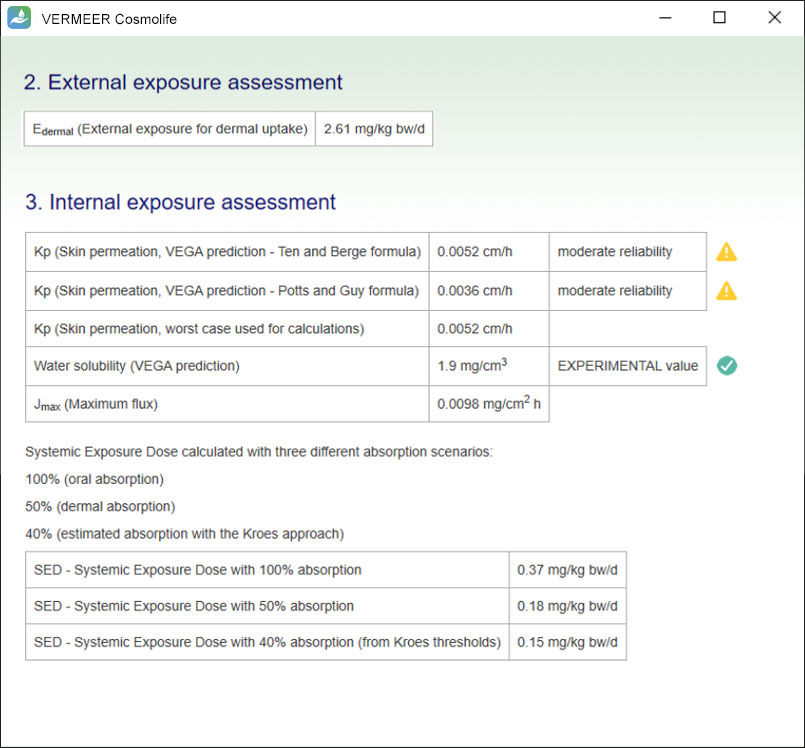
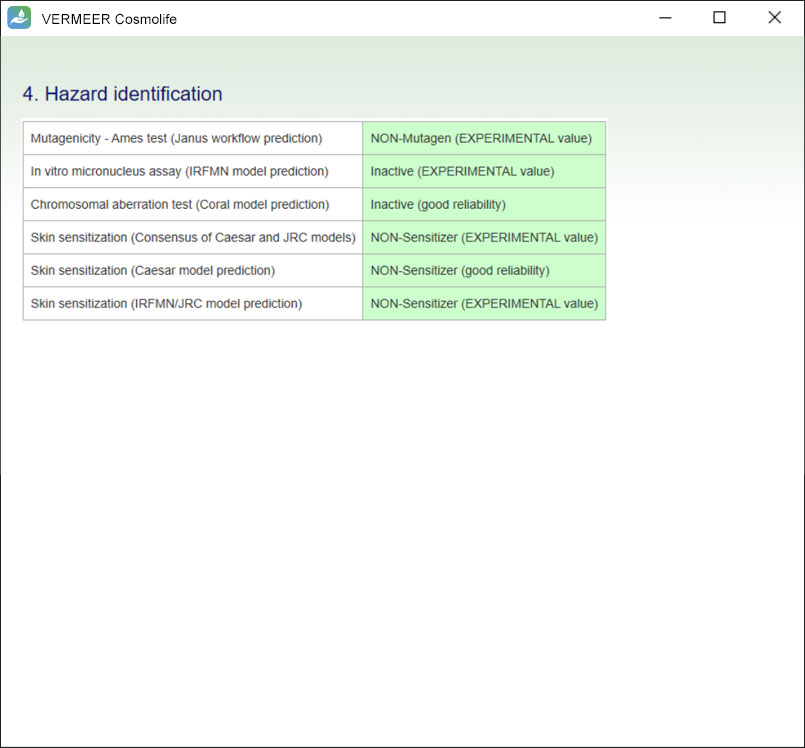
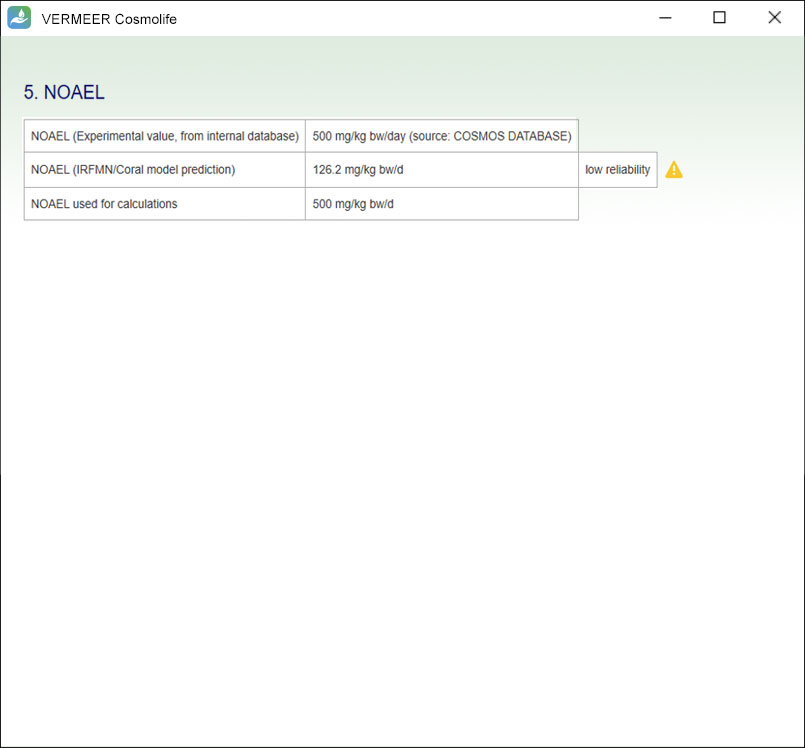
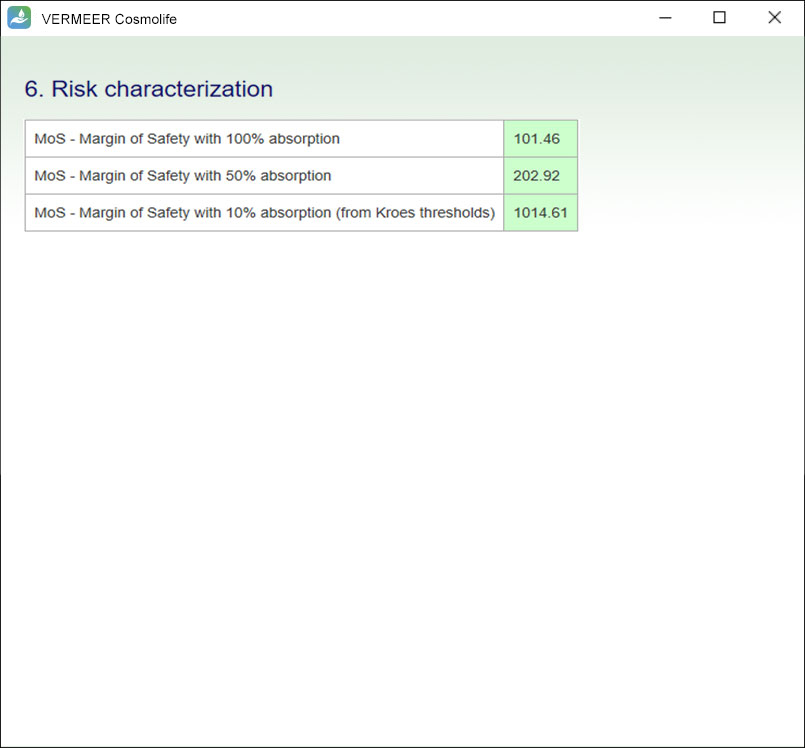
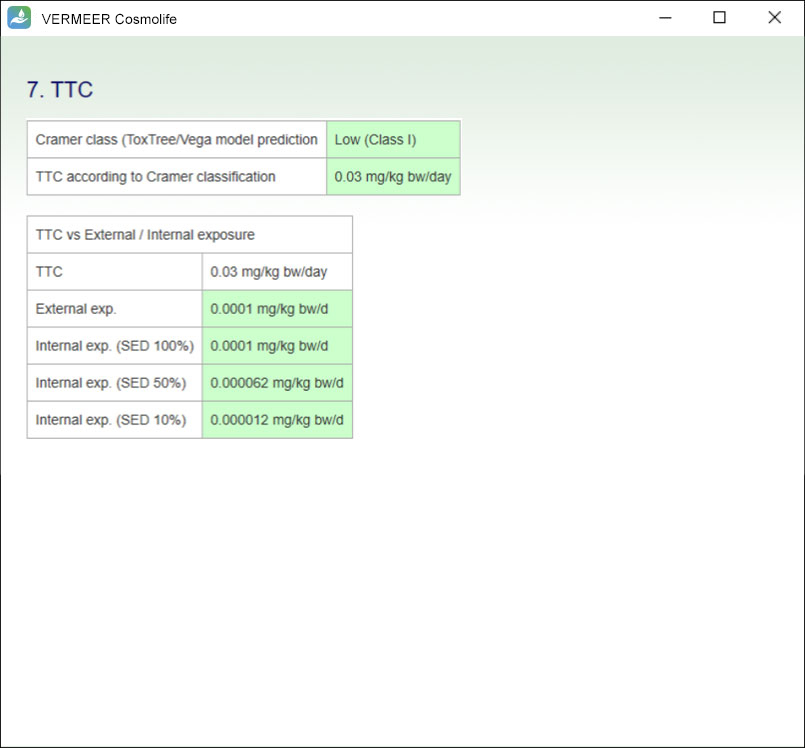
The focus is on systemic toxicity and the Margin of Safety (MoS) is calculated, considering the No Observed Adverse Effect Level (NOAEL). It includes the assessment considering the Threshold of Toxicological Concern (TTC) approach proposed by Williams (Williams et al., 2016), and a number of tools for mutagenicity, genotoxicity and skin sensitization. Other endpoints will be added in the future.
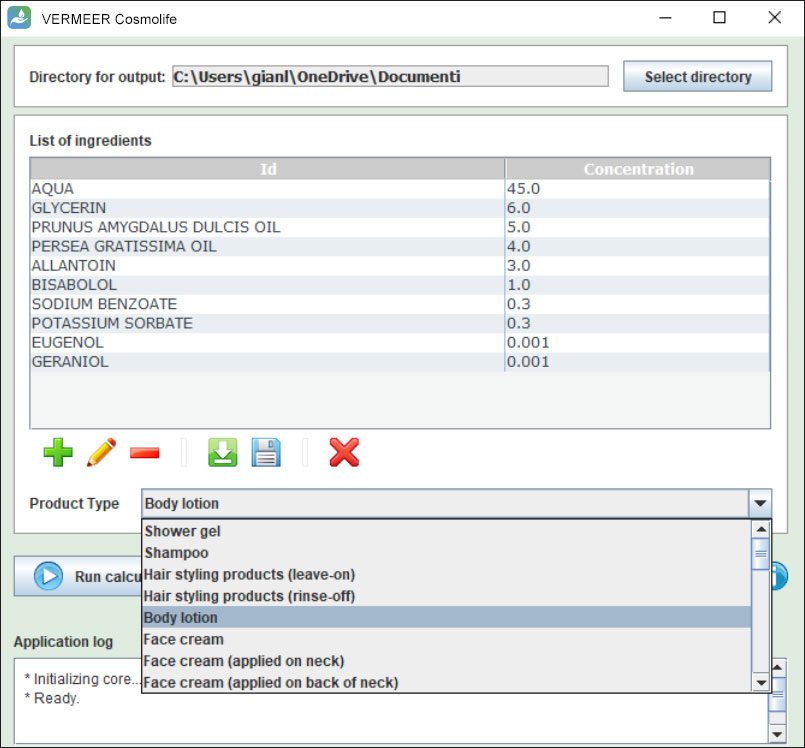
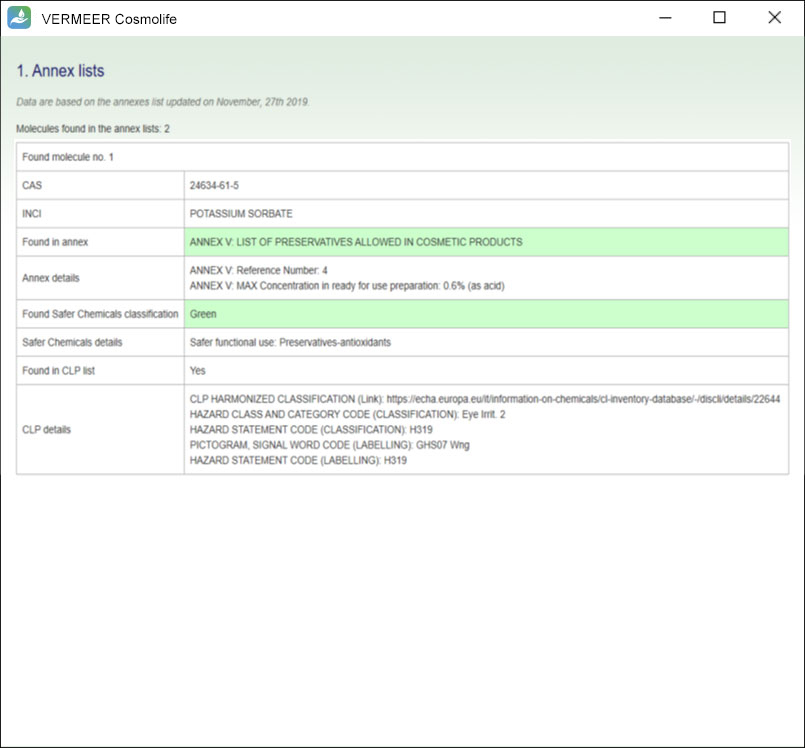
The software can process multiple ingredients in a product, and make predictions, in case of missing experimental values. The user is asked to provide the information regarding the ingredients, their concentration and the product type. The software checks if the ingredient is present in the Annexes of the “Regulation (EC) No. 1223/2009 of the European parliament and of the Council of 30 November 2009 on cosmetic products”. Then, the software searches for values present in its database useful for TTC, genotoxicity and skin sensitization. Moreover, the software allows comparing the external and internal exposure, using a specific model for skin permeation.
Free download VERMEER Cosmolife Software
Visit the link to download the application.